The Silver Figurehead - Part II
by Joseph A. A. Bourque Sr.
Issue 305 - September 1998
Dear Reader,
Two months ago I was able to share my Silver Lady Sea Shell, and/or Line No. 3011 flower bowl or fruit bowl, with you. I attended our Silver Anniversary Convention. I brought her along with me, as Chairman Mark Nye had requested members to bring along unusual silver decorated items needed for a display. Some members requested I bring along certain items I had written articles about, so they could see them. Rather than just bring along certatn pieces, I decided to bring along each piece that I had written an article on during the past year.
 Upon arriving at the convention I left the Silver Lady with Chairman
Nye, and he placed her on display. I received the Chairman's permission
to display the other articles had brought along on a table in front of
my motel room during the picnic. They received a lot of attention and
kept me busy for four hours.
Upon arriving at the convention I left the Silver Lady with Chairman
Nye, and he placed her on display. I received the Chairman's permission
to display the other articles had brought along on a table in front of
my motel room during the picnic. They received a lot of attention and
kept me busy for four hours.
My wife and I enjoyed the Convention very much, and wish to convey a heartfelt thank you from both of us to Chairman Mark Nye and his Convention committee for a job well done. In spite of the unusual weather, Mr. Nye saw to it that each indoor event took place, and on time.
For those of you who were unable to attend the Convention, I will try my best to relate the salient facts delivered to us by the most learned person on Silver Decorated Glass, doctor and professor David E. Fairbrothers, who was the Silver Anniversary Convention speaker.
In his opening statement, learned Dr. Fairbrothers listed the various names given to silver decorated glass such as: Overlay, Applique, Resist, Plate, Lustre, Coated, Gilded, Leafed, etc., none which really apply. The correct name to use is: Silver Deposit.
Dr. Fairbrothers was asked: "What should be done to care for this Silver Deposit Glass?" His reply "They say nothing should be done in the line of polishing or shining it up". He did not say that he agrees or disagrees with their policy, but that he elects to polish his pieces. He was asked for the second time by the same NCC member as to what should be done in the line of care. Several people were speaking and asking questions at the same time end the question went on unanswered.
At this point, I got the doctor's attention and said: "Doctor, I own some Cambridge Siliver Deposit items that have turned black-blue from years of oxidation. I am the type of collector who does not believe in taking away any of the old patina by polishing or shining it." His answer was delivered to me loud, clear, and very quickiy as he said: "By all means, don't do anything to it it you wish to keep it that way." Then he added: "Understand, however, that it is not the oxygen in the atmosphere that darkens the silver, it is the sulfur in the air that causes silver to turn black." He went on to say: "To maintain the old look wash it with a soft brush in a mild detergent solution, rinse it off well, and pat it dry with a soft absorbent cloth."
My next statement/question to the college professor with the doctorate was: "I have been informed by more than one person that if I allow the age-old patina to remain, it will eventually become pitted. Could you please comment on this?" At this point dear readers, pay very close attention to this wise mans answer, as it is classic. He said: "lt is not leaving the tarnish on that will cause pitting on your silver, it is the process of using silver polish on your silver and not cleaning it off properly that will cause the pitting. Remember this: Silver polish oontains acid, and acid causes pitting. After polishing, make sure that you remove all of the polish."
The doctor gave another bit of good advice. When polishing your Silver Deposit Glass (should you elect to do so), never polish it nor buff it off with cloth, as the threads of the cloth will get caught under the sharp or fine points of the silver design and will cause them to pull up and break. How true this is. I have noted this damage time and time again.
The doctor shared some more of his knowledge by explaining how Silver Deposit was placed on glass objects. This subject was most interesting, and I will make an attempt to explain the system strictly from a layman's viewpoint. Suffice it to say that a flux containing silver particles and a gluing agent is made that will allow electricity to flow through it. The design to be made is drawn or stenciled on the glass with the flux, and is allowed to dry and become glued to a glass item. A silver ingot is connected to the one electrical direct current anode that will flow to the other anode. A conduit wire is connected to the ingot and one end of the silver flux design. A conduit wire from the opposite end of the design is wired to the opposite DC anode. The item containing the silver flux design along with the silver ingot are completely submerged in an acid bath.
The electrolysis process then begins. The acid bath starts to eat into the silver ingot. The electrical flow carries the silver particles being discharged trom the ingot to the silver flux design on the glass where they become attached to the silver in the flux or the design to be silvered. After approximately four hours, enough silver is taken from the silver ingot to hecome attached to the silver flux to form a choice Silver Deposit design.
The speaker also made mention of a special type of silvering to glass that never needs cleaning, the Silver Deposit is applied in the regular manner. The silver ingot is then replaced with a platinum ingot. This causes the Silver Deposit decoration to become coated or plated with PLATINUM, which will never tarnish. This process is rarely found, however, because it is quite expensive to make.
The doctor explained that before buying a piece of Silver Deposit, a close examination ought to be conducted paying close attention to the condition of all of the edges of the design, to ensure that none of the edges are "erode," a condition that shows edge damage because of improper workmanship. All edges of the silver should be tight and "giued" to the glass. He stated that he would never buy an "erode" piece of Silver Deposit, as the condition worsens with age.
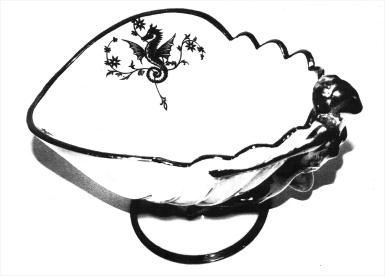
The sage professor, when finished with his lecture, gave us some extra time by answering some of our personal questions. I showed him the front page of the July issue of the Crystal Ball depicting the Flying Silver Lady and one of the two Silver Deposit seahorse designs. (It may have been my imagination, but I felt he enjoyed being called professor as opposed to being addressed as a doctor). "Professor, I addressed him "To whom do you attribute the making of this seahorse design?" I was as tense as the main mast of a triple- masted schooner in an Atlantic squall. Without hesitation he said: "Rockwell." "Thank you, Professor," said I. (He had agreed with my own personal silent opinion.)
I was elated. -- The Triangle-C jitters came and went, and I actually became thrilled to think that my Fathers' Day gift from my lovely daughter, Darlene, was a gem of gems in the 3011 Line or the Sea Shell Line, and that the maker of the Silver Deposit was the work of The Dean of Glass Silvering, namely ROCKWELL.
It was at this point that I heard a voice state: "the Doctor was correct. Those seahorse designs were made by Rockwell." I turned around to see the face of the voice that was speaking. It was that of an old acquaintance, Willard Kolb, whom I first met at an NCC convention in the mid-1970s. We greeted each other. I then asked him how he knew that this seahorse silver decoration was a Rockwell product. In his hands, he actually held two small Crown Tuscan Sea Shell plates with Charleton decorations bearing the exact design of the seahorse decoration also in Silver Deposit. He simply said, "take a look," as he handed me one of the plates in an inverted position. The base of the plate bore the Rockwell signature in acid etching. At this point I cannot express my feeling how everything fell into place so perfectly.
Dear Readers, take a good look at the seahorse design and remember what it looks like. You too may become a proud owner of a Rockwell Silver Deposit Cambridge Glass item.
Until next time,
Joe
